

The FDA labeling that states that NETSPOT is a radioactive diagnostic agent indicated for use. Radioactive diagnostic agent after radiolabeling with Ga 68 indicated for use with positron-emission tomography (PET) for localization of somatostatin receptor-positive neuroendocrine tumors (NETs) in adults and children. National Center for Biotechnology Information 8600 Rockville Pike, Bethesda, MD, 20894 USA Contact Policies FOIA HHS Vulnerability Disclosure National Library of Medicine National Institutes of Health Department of Health and Human Services USA.
#Netspot fda registration#
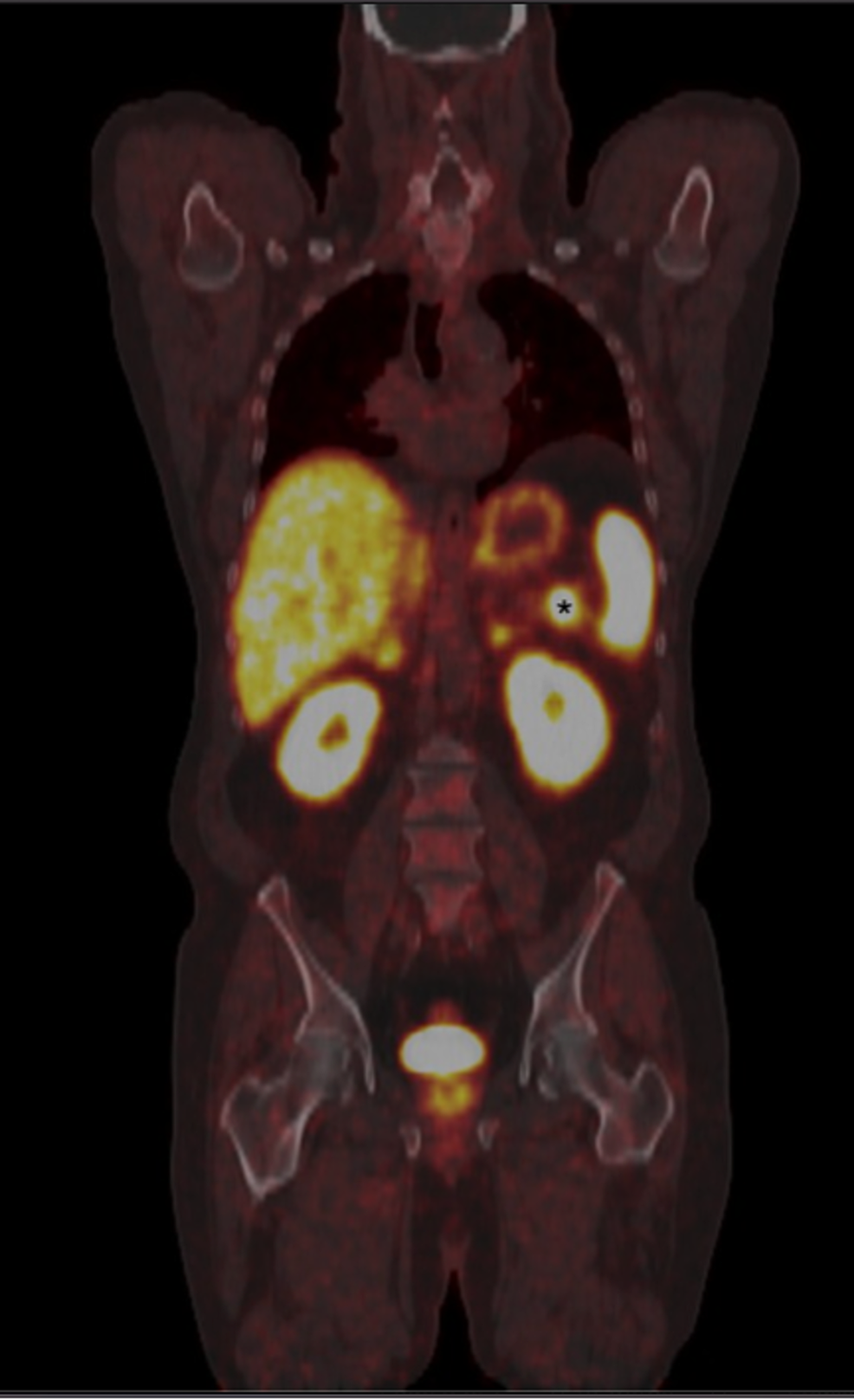
If you opt-out your email will still be collected for registration purposes. Gallium Ga 68 Dotatate PET (NetSpot) for Neuroendocrine Tumors. You have the right to opt-out of sharing your email address with your organization but doing so may negatively affect your organization’s decision to renew their subscription to AdisInsight. Gallium 68 NETSPOT, an FDA approved PET drug used for neuroendocrine tumor. FDA-approved in 2016, NETSPOT is used in imaging to locate somatostatin receptor positive neuroendocrine tumors in adult and pediatric patients. To be approved by the FDA, the generic version must deliver the same amount. The number of times you access AdisInsight, the number of searches you performed, and the number of profiles you viewed will be provided to your organization both in aggregate with other users and individually by your email address. NETSPOT (68Ga-Dotatate) is a radioactive diagnostic agent used for PET imaging that is gaining ground among practices. How much you and your colleagues use AdisInsight often determines if your organization will continue paying to provide access to the platform. 8f4f1169-c7bd-47b8-b85e-d8243394651d.pdf PRESS RELEASE Advanced Accelerator Applications Announces FDA Approval of NETSPOTTM, a Kit for the Preparation of Gallium. By accessing or using the AdisInsight platform you agree to the terms of use.


 0 kommentar(er)
0 kommentar(er)
